- As a single agent or in combination for the first line treatment of stage 3 or 4 non-small cell lung cancer.
- Treatment of advanced breast cancer stage 3 and 4 relapsing after or refractory to an anthracycline containing regimen.
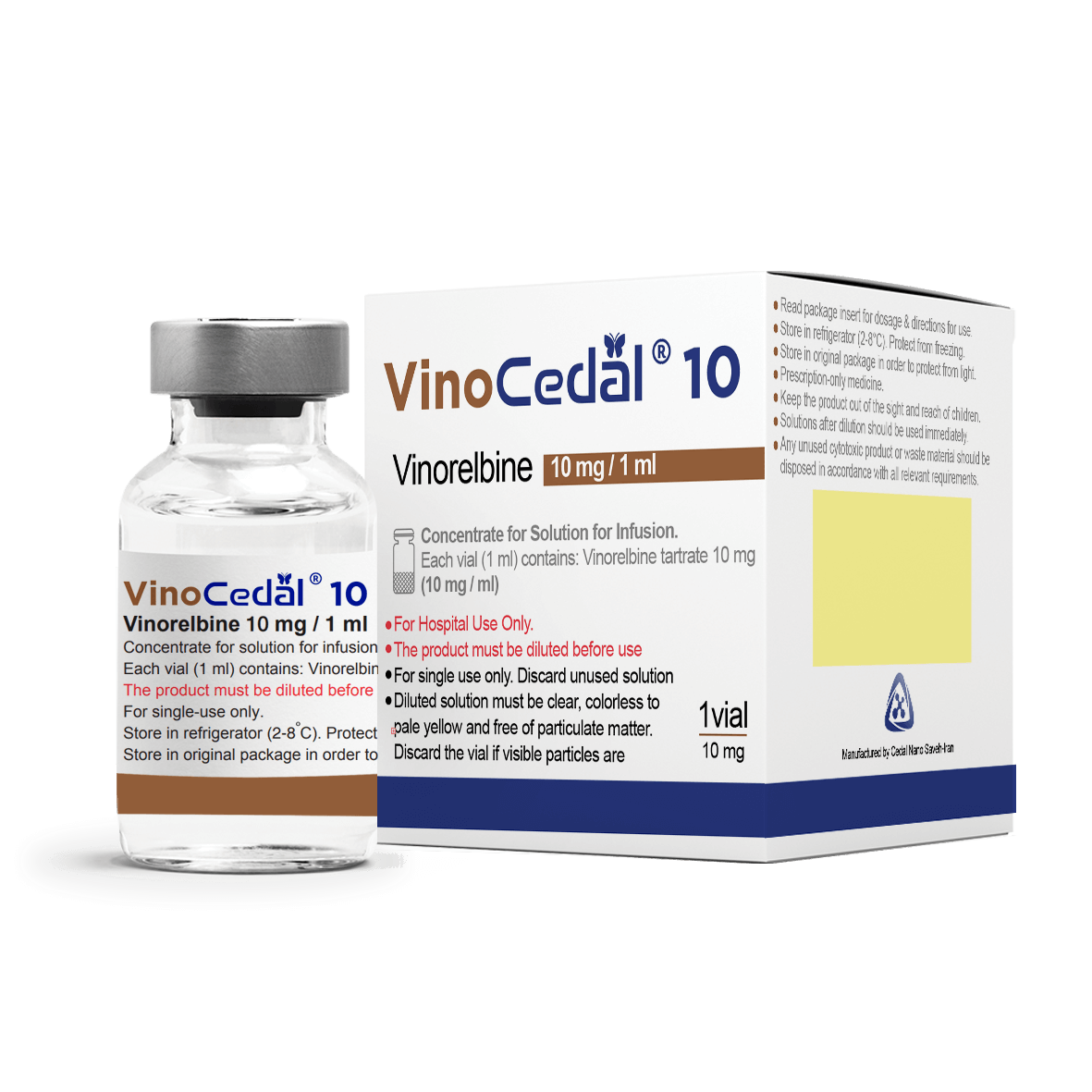
VinoCedal®
Generic Name
Brand Name
Drug Class
Dosage Form
Indications
Contradications
Warnings and Precautions
Dosage & Administration
Adverse Reactions
Drug Interactions
Pregnancy and Lactation
Storage Conditions
Indications
Contradications
- Hypersensitivity to the active substance or other vinca alkaloids, or to any of the excipients
- Neutrophil count < 1,500/mm3 or severe current or recent infection (within the last 2 weeks)
- Thrombocyte count below 100,000/mm3
- Severe hepatic impairment not related to the tumoural process
- In combination with yellow fever vaccine
- Pregnancy
Warnings and Precautions
Special warnings
- VINOCEDAL® should be administered under the supervision of a physician experienced in the use of chemotherapy.
- VINOCEDAL® must only be administered by the intravenous route. The use of intrathecal route is contra-indicated. Administration should always be followed by a sodium chloride 9 mg/ml (0.9 %) infusion to flush the vein.
VINOCEDAL® must be administered intravenously with great precision: It is very important to make sure that the cannula has been accurately placed into the vein before starting to infuse VINOCEDAL®. If VINOCEDAL® extravasates during intravenous administration, this can cause considerable local irritation. In this case, the infusion must be stopped immediately, the vein flushed through with sodium chloride 9 mg/ml (0.9 %) solution and the rest of the dose should be administered in another Additionally, published data support the use of treatment with hyaluronidase and dry heat in the event of conservative treatment is recommended.
- Treatment should be undertaken with close haematological monitoring (determination of haemoglobin level and number of leukocytes, granulocytes and thrombocytes before each new injection). The dose-limiting adverse reaction is mainly This effect is non-cumulative, having its nadir between 7 and 14 days after the administration and is rapidly reversible within 5 to 7 days. If the neutrophil count is <1,500/mm3and/or thrombocyte count is below 100,000/mm3, treatment should be delayed until recovery and the patient should be observed. Administration of the medicinal product is expected to be delayed by 1 week in about 35% of treatment courses.
- If patients present signs or symptoms suggestive of infection, a prompt investigation should be carried out.
- Interstitial lung disease has been reported more frequently in the Japanese Special attention should be exercised for this specific population. Special precautions for use
- If there is significant hepatic impairment the dose should be reduced: caution is recommended and careful monitoring of haematological parameters required.
- In case of renal impairment, because of the low level of renal excretion, no dose modification is necessary.
- VINOCEDAL® should not be given concomitantly with radiotherapy if the treatment field includes the liver.
- Strong CYP3A4 inhibitors or inducers should be administered with caution because of the risk of affecting the VINOCEDAL® concentration.
- This product is generally not recommended in combination with itraconazole (like all vinca alkaloids) and phenytoin (like all cytotoxics).
- This product is specifically contraindicated with yellow fever vaccine and its concomitant use with other live attenuated vaccines is not recommended.
- To avoid bronchospasm – especially if used concomitantly with mitomycin C-appropriate precautionary measures should be Patients treated on an outpatient basis should be informed that they should contact the physician in case of dyspnoea.
- It is recommended that special caution should be shown towards patients with ischaemic heart disease in the medical history
- All contact with the eyes should be strictly avoided: risk of severe irritation and even corneal ulceration if the medicinal product is sprayed under Immediate liberal washing of the eye with sodium chloride 9 mg/ml (0.9 %) solution should be undertaken if any contact occurs.
Dosage & Administration
Dosage:
VINOCEDAL® is usually given at 30-25 mg/m2 once weekly.
In combination with other cytostatic agents the exact dose should be taken from the treatment protocol.
VINOCEDAL® may be administered by slow bolus (6-10 minutes) after dilution in 20-50 ml of sodium chloride 9 mg/ml (0.9 %) solution for injection or in 5 % (w/v) glucose solution for injection or by a short infusion (20-30 minutes) after dilution in 125 ml of sodium chloride 9 mg/ml (0.9 %) solution for injection or in 5 % (w/v) glucose solution for injection. Administration should always be followed by a sodium chloride 9 mg/ml (0.9 %) infusion with at least 250 ml to fush the vein.
The maximum tolerated dose per administration: 35.4 mg/m2 body surface area
The maximum total dose per administration: 60 mg
Dose Adjustment Recommendations
Vinorelbine metabolism and clearance are mostly hepatic: only 18.5 % is excreted unchanged in the urine. No prospective study relating altered metabolism of the active substance to its pharmacodynamic effects is available in order to establish guidelines for vinorelbine dose reduction in patients with impaired liver or kidney function.
Hepatic impairment
The pharmacokinetics of vinorelbine is not modified in patients presenting moderate or severe live impairment.
Nevertheless as a precautionary measure a reduced dose of 20 mg/m2 and close monitoring of haematological parameters is recommended in patients with severe liver impairment.
Renal impairment
Given the minor renal excretion, there is no pharmacokinetic rationale for reducing vinorelbine dose in patients with impaired kidney function.
Elderly
Clinical experience has not detected any significant differences among elderly patients with regard to the response rate, although greater sensitivity in some of these patients cannot be excluded. Age does not modify the pharmacokinetics of vinorelbine.
Paediatric population
The safety and efficacy in children have not been established an administration is therefore not recommended.
Method of Administration:
Strictly intravenous administration after appropriate dilution.
Intrathecal administration of vinorelbine may be fatal.
Precautions to be taken before handling or administering the medicinal product
For intravenous administration via syring, the calculated dose of vinorelbine tartrate is diluted to a concentration of 1.5 to 3 mg/mL with either %5 dextrose injection or %0.9 sodium chloride injection.
For administartion via an intravenous bag, the calculated dose of vinorelbine tartrate injection is diluted to a concentration of 0.5 to 2 mg/mL with %5 dextrose injection, 0.9 % sodium chloride injection, 0.45 % sodium chloride injection, 5 % dextrose in 0.45 % sodium chloride injection, Ringer’s injection or lactated Ringer’s injection.
— Administration should always be followed with at least 250 mL of a normal saline infusion to flush the vein.
Adverse Reactions
Like all medicines, this medicine can cause side effects, although not everybody gets them. Side effects with VINOCEDAL® may include:
• >10%:
Central nervous system: Fatigue (%27), peripheral neuropathy (%25; grade :3 %1; grade %1> :4)
Dermatologic: Alopecia (%12 to %30), Gastrointestinal: Nausea (%31 to %44; grade %1 :3 to %2), constipation
(%35; grade %3 :3), vomiting (%20 to %31; grade %1 :3 to %2), diarrhea (%12 to %17)
Hematologic & oncologic: Leukopenia (%83 to %92; grade %6 :4 to %15), granulocytopenia (%90; grade %36 :4; nadir: 7 to 10 days; recovery 14 to 21 days), neutropenia (%85; grade %28 :4), anemia (%83; grades %9 :4/3) Hepatic: Increased serum AST (%67; grade %5 :3; grade %1 :4), increased serum bilirubin (total bilirubin: %5 to %13; grade %4 :3; grade %3 :4)
Local: Injection site reaction (%22 to %28; includes erythema at injection site, vein discoloration), pain at injection site (%16)
Neuromuscular & skeletal: Weakness (%36) Renal: Increased serum creatinine (%13)
• 1-10%:
Cardiovascular: Localized phlebitis (%7 to %10), chest pain (%5) Central nervous system: Decreased deep tendon reflex (<%5) Dermatologic: Skin rash (<%5)
Gastrointestinal: Paralytic ileus (%1)
Hematologic & oncologic: Febrile neutropenia (≤%8; grade %4≥ :4), thrombocytopenia (%3 to %5; grades %1 :4/3)
Infection: Sepsis (≤%8; grade %4≥ :4)
Neuromuscular & skeletal: Arthralgia (<%5), myalgia (<%5), jaw pain (<%5)
Otic: Ototoxicity (≤%1) Respiratory: Dyspnea (%7)
• <1%:
Postmarketing, and/or case reports: Abdominal pain, anaphylaxis, angioedema, back pain, deep vein thrombosis, dysphagia, esophagitis, ushing, headache, hemolytic-uremic syndrome, hemorrhagic cystitis, hypersensitivity reaction, hypertension, hyponatremia, hypotension, intestinal necrosis, intestinal obstruction, intestinal perforation, interstitial pulmonary disease, ischemic heart disease, localized rash, mucositis, myasthenia, myocardial infarction (rare), pancreatitis, pneumonia, pruritus, pulmonary edema, pulmonary embolism, radiation recall phenomenon (dermatitis, esophagitis), skin blister, SIADH (syndrome of inappropriate antidiuretic hormone secretion), tachycardia, thromboembolism, thrombotic thrombocytopenic purpura, tumor pain, unsteady gait, urticaria, urticaria at injection site, vasodilatation
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Please report any adverse drug reactions via contacting Cedal Nano
Drug Interactions
Interactions common to all cytotoxics
Due to the increase of thrombotic risk in case of tumoural diseases, the use of anticoagulative treatment is frequent. If the patient receives anticoagulative treatment the frequency of INR (International Normalised Ratio) monitoring should be increased, due to high intra-individual variability of the coagulability during diseases, and the eventuality of interaction between oral anticoagulants and anticancer chemotherapy.
Concomitant use no recommended
This product is generally not recommended in combination with live attenuated vaccines because of the risk of generalised, possibly fatal vaccine disease. This risk is increased in patients already immunodepressed by their underlying disease. It is recommended to use an inactivated vaccine when exists (poliomyelitis).
Concomitant use contraindicated
phenytoin digestive absorption by the cytotoxic medicinal product or risk of toxicity enhancement or loss of efficacy of the cytotoxic medicinal product due to increased hepatic metabolism by phenytoin.
Concomitant use to take into consideration
Ciclosporine, tacrolimus: Excessive immunosuppression with risk of lymphoproliferation is to be taken into consideration.
Interactions specific to vinca alkaloids
Concomitant use not recommended
Itraconazole should not be administered concomitantly because of the risk of increased neurotoxicity due to the decrease of their hepatic metabolism.
Concomitant use to take into consideration
Concomitant use of vinca alkaloids and mitomycin C increases the risk of bronchospasm and dyspnoea. In rare cases, particularly in combination with mitomycin, an interstitial pneumonitis was observed.
Vinorelbine is a P-glycoprotein substrate and concomitant use with inhibitors (e.g. verapamil, ciclosporin and quinidine) or inducers of this transport protein can affect the concentration of vinorelbine.
Interactions specific to vinorelbine
The combination of vinorelbine with other medicinal products with known bone marrow toxicity is likely to exacerbate the myelosuppressive adverse reactions.
As CYP 3A4 is mainly involved in the metabolism of vinorelbine, combination with strong inhibitors of this isoenzyme (e.g. itraconazole, ketoconazole, clarithromycin, erythromycin and ritonavir) could increase blood concentrations of vinorelbine and combination with strong inducers of this isoenzyme (e.g. rifampicin, phenytoin, phenobarbital, carbamazepin and St. John’s wort) could decrease blood concentrations of vinorelbine.
The combination of vinorelbine and cisplatin (a very common combination) does not affect the pharmacokinetic parameters. However, there is higher incidence of granulocytopenia in the combination of vinorelbine and cisplatin than in vinorelbine as monotherapy.
An increased incidence of grade 3/4 neutropenia has been suggested when intravenous vinorelbine and lapatinib were associated in one clinical phase I study. In this study, the recommended dose of intravenous form of vinorelbine in a 3-weekly schedule on day 1 and day 8 was 22.5 mg/m2 when combined with daily lapatinib 1000 mg. This type of combination should be administered with caution.
Pregnancy and Lactation
Pregnancy
There are no or limited amount of data from the use of vinorelbine in pregnant women. Animal studies have shown embryotoxicity and teratogenicity. Based on the results of animal studies and the pharmacological action of vinorelbine, this medicinal product is suspected to cause congenital malformations when administered during pregnancy. VINOCEDAL® is contraindicated during pregnancy. Women should not become pregnant during treatment with VINOCEDAL®. In case of a vital indication a medical consultation concerning the risk of harmful effects for the child should be performed for the therapy of a pregnant patient. If pregnancy occurs during the treatment, the possibility of genetic counselling should be considered.
Breast feeding
It is unknown whether vinorelbine is excreted in human milk. The excretion of vinorelbine in milk has not been studied in animals. A risk to the newborns/infants cannot be excluded. Breast-feeding must be discontinued before starting treatment with VINOCEDAL®.
Storage Conditions
Shelf life
In unopened packaging: 2 years.
After opening and dilution:
The medicinal product has to be used immediately after opening and dilution. For single dose only.
From a microbiological point of view, the product should be used immediately.
Storage
Store in refrigrator(2-8 °C) .Protect from freezing.
Store in original package in order to protect from light.
The diluted solution in 0.9% sodium chloride and 5% dextrose should be used immediately.